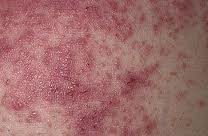
Dermatitis herpetiformis (DH), or Duhring’s disease, is a chronic blistering skin condition, characterised by blisters filled with a watery fluid. Despite its name, DH is not related to or caused by herpes virus: the name means that it is a skin inflammation having an appearance similar to herpes.
 DH was first described by Dr. Louis Duhring in 1884. A connection between DH and gluten intolerance (coeliac disease) was recognised in 1967, although the exact causal mechanism is not known.
DH was first described by Dr. Louis Duhring in 1884. A connection between DH and gluten intolerance (coeliac disease) was recognised in 1967, although the exact causal mechanism is not known.
The age of onset is usually about 15-40, but DH can also affect children and the elderly. Men and women are equally affected. Estimates of DH prevalence vary from 1 in 400 to 1 in 10000.
Symptoms
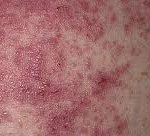
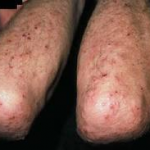
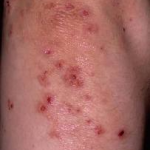
Dermatitis herpetiformis is characterized by intensely itchy, chronic papulovesicular eruptions, usually distributed symmetrically on extensor surfaces (buttocks, back of neck, scalp, elbows, knees, back, hairline, groin, or face). The blisters vary in size from very small up to 1 cm across.
The condition is extremely itchy, and the desire to scratch can be overwhelming. This sometimes causes the sufferer to scratch the blisters off before they are examined by a doctor. Intense itching or burning sensations are sometimes felt before the blisters appear in a particular area.
Untreated, the severity of DH can vary significantly over time, in response to the amount of gluten ingested.
Dermatitis herpetiformis symptoms typically first appear in the early years of adulthood between 20 and 30 years of age.
Although the first signs and symptoms of dermatitis herpetiformis are intense itching and burning, the first visible signs are the small papules or vesicles that usually look like red bumps or blisters. Sometimes they appear on the face and along the hairline, and, on occasion, on the shoulders, the lower end of the spinal column, and within the mouth. The rash rarely occurs on other mucous membranes, excepting the mouth or lips. The symptoms range in severity from mild to serious, but they are likely to disappear if gluten ingestion is avoided and appropriate treatment is administered.
Dermatitis herpetiformis symptoms are chronic, and they tend to come and go, mostly in short periods of time. Sometimes, these symptoms may be accompanied by symptoms of coeliac disease, commonly including abdominal pain bloating or loose stool and fatigue.
The rash caused by dermatitis herpetiformis forms and disappears in three stages. In the first stage, the patient may notice a slight discoloration of the skin at the site where the lesions appear. In the next stage, the skin lesions transform into obvious vesicles and papules that are likely to occur in groups. Healing of the lesions is the last stage of the development of the symptoms, usually characterized by a change in the skin color. This can result in areas of the skin turning darker or lighter than the color of the skin on the rest of the body. Because of the intense itching, patients usually scratch, which can lead to the formation of crusts.
Pathophysiology
Dermatitis herpetiformis is a disease of the skin caused by the deposition of IgA in the papillary dermis, which triggers an immunologic cascade, resulting in neutrophil recruitment and complement activation. Dermatitis herpetiformis is the result of an immunologic response to chronic stimulation of the gut mucosa by dietary gluten.
An underlying genetic predisposition to the development of dermatitis herpetiformis has been demonstrated. Both dermatitis herpetiformis and celiac disease (CD) are associated with an increased expression of HLA-A1, HLA-B8, HLA-DR3, and HLA-DQ2 haplotypes. Environmental factors are also important; monozygotic twins may have dermatitis herpetiformis, celiac disease, and/or gluten-sensitive enteropathy with variable symptomatology.
The leading theory for dermatitis herpetiformis is that a genetic predisposition for gluten sensitivity, coupled with a diet high in gluten, leads to the formation of IgA antibodies to gluten-tissue transglutaminase (t-TG), which is found in the gut. These antibodies cross-react with epidermal transglutaminase (e-TG).eTG is highly homologous with tTG. Serum from patients with gluten-sensitive enteropathy, with or without skin disease, contains IgA antibodies to both skin and gut types.Deposition of IgA and epidermal TG complexes in the papillary dermis cause the lesions of dermatitis herpetiformis.
In patients with gluten-sensitive enteropathy, levels of circulating antibodies to tissue and epidermal transglutaminase have been found to correlate with each other, and both appear to correlate with the extent of enteropathy.
Co-localized IgA and eTG deposits have been demonstrated in the papillary dermis in patients with dermatitis herpetiformis and, to lesser extent, in healthy skin of gluten-sensitive enteropathy patients.eTG has not been demonstrated in normal papillary dermis, suggesting it is part of the circulating complex that is deposited in the papillary dermis, rather than originating from the papillary dermis.
 Cutaneous IgA deposits in dermatitis herpetiformis have been shown to function in vitro as a ligand for neutrophil migration and attachment. Although IgA deposition is pivotal for disease, an increased serum IgA is not necessary for pathogenesis; in fact, case reports describe dermatitis herpetiformis in patients with a partial IgA deficiency.When the disease is active, circulating neutrophils have a higher level of CD11b and an increased ability to bind IgA. The characteristic histologic finding of dermatitis herpetiformis is neutrophil accumulation at the dermoepidermal junction, frequently localizing to the papillary tips of the basement membrane zone.
Cutaneous IgA deposits in dermatitis herpetiformis have been shown to function in vitro as a ligand for neutrophil migration and attachment. Although IgA deposition is pivotal for disease, an increased serum IgA is not necessary for pathogenesis; in fact, case reports describe dermatitis herpetiformis in patients with a partial IgA deficiency.When the disease is active, circulating neutrophils have a higher level of CD11b and an increased ability to bind IgA. The characteristic histologic finding of dermatitis herpetiformis is neutrophil accumulation at the dermoepidermal junction, frequently localizing to the papillary tips of the basement membrane zone.
Collagenase and stromelysin 1 may be induced in basal keratinocytes either by cytokines released from neutrophils or by contact with keratin from damaged basement membrane matrix. Stromelysin 1 may contribute to blister formation.
One study found levels of E-selectin mRNA expression in normal-appearing skin of patients with dermatitis herpetiformis to be 1271 times greater that that of controls.Additionally, the same study observed increased soluble E-selectin, IgA antitissue transglutaminase antibodies, tumor necrosis factor-alpha, and serum interleukin 8 (IL-8) levels in patients with dermatitis herpetiformis, providing further evidence of endothelial cell activation and a systemic inflammatory response as part of the pathogenic mechanism of the disease. Mild local trauma may also induce the release of cytokines and attract the partially primed or activated neutrophils, which is consistent with the typical location of dermatitis herpetiformis lesions on frequently traumatized areas, such as the knees and elbows.
Deposits of C3 also may be present in a similar pattern at the dermoepidermal junction. The membrane attack complex, C5-C9, also has been identified in perilesional skin, although it may be inactive and not contribute to cell lysis.
A recent study showed an increased expression of disintegrin and metalloproteinase (ADAMs) 8, 10, 15, and 17 in lesional skin of patients with dermatitis herpetiformis compared with controls. The high affinity of ADAMs for the basement membrane led the authors to hypothesize a role in blister formation in dermatitis herpetiformis.
Hormonal factors may also play a role in the pathogenesis of dermatitis herpetiformis, and reports describe dermatitis herpetiformis induced by treatment with leuprolide acetate, a gonadotropin-releasing hormone analog.Androgens have a suppressive effect on immune activity, including decreased autoimmunity, and androgen deficient states may be a potential trigger for dermatitis herpetiformis exacerbation. Exacerbation of dermatitis herpetiformis by oral contraceptives has also been reported.
 Apoptosis may contribute to the pathogenesis of epidermal changes in dermatitis herpetiformis, and research demonstrates a markedly increased apoptotic rate within the epidermal compartment in dermatitis herpetiformis.In addition, Bax and Bcl-2 proteins are increased in the dermal perivascular compartment and Fas proteins showed epidermal staining in dermatitis herpetiformis lesions.
Apoptosis may contribute to the pathogenesis of epidermal changes in dermatitis herpetiformis, and research demonstrates a markedly increased apoptotic rate within the epidermal compartment in dermatitis herpetiformis.In addition, Bax and Bcl-2 proteins are increased in the dermal perivascular compartment and Fas proteins showed epidermal staining in dermatitis herpetiformis lesions.
Most patients with dermatitis herpetiformis have histologic evidence of enteropathy, even in the absence of symptoms of malabsorption. In one study, all dermatitis herpetiformis patients had increased intestinal permeability (as measured by the lactulose/mannitol ratio) and up-regulation of zonulin, a regulator of tight junctions.Thus, increased expression of zonulin may be involved in the pathogenesis of enteropathy in patients with dermatitis herpetiformis.